Skip to content
All of the Important Information!
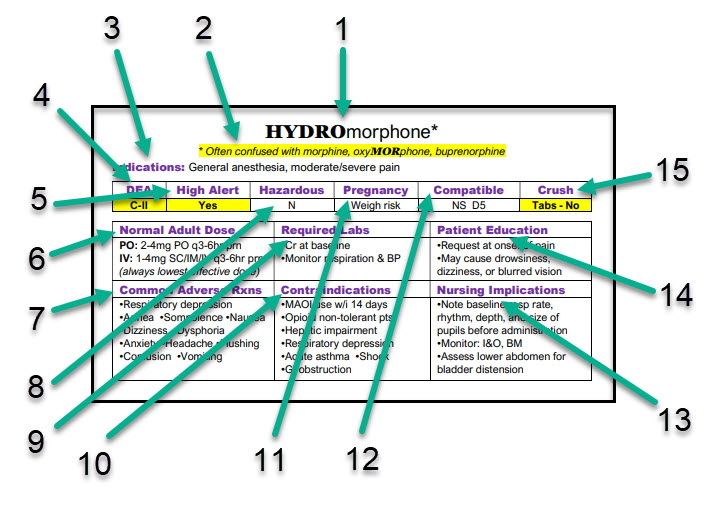
- 1 – The Generic Name of the Drug with tallMAN Lettering (where appropriate): tallMAN lettering is special labeling from The Institute for Safe Medical Practices (ISMP) for look-a-like drug names. It is used on drug labels and electronic drug orders to help prevent medical errors.
- 2 – Look-alike / Sound-alike Drug Names: The Institute for Safe Medical Practices (ISMP) identifies drugs that have a high error rate involving confusion of drugs with similar names. (appears when appropriate)
- 3 – Approved Indications: Identifies common uses for the drug.
- 4 – DEA Schedule: Identifies if a drug is classified as a controlled substance by the Drug Enforcement Administration (DEA). If it is, the schedule is indicated.
- 5 – High Alert: Identifies drugs which the Institute for Safe Medical Practices (ISMP) has listed as requiring greater attention from the worker to prevent medical errors that may injure the patient.
- 6 – Adult Dose: Lists the published dose for adult patients without other mitigating health conditions
- 7 – Common Adverse Reactions: A list of commonly seen adverse effects in patients.
- 8 – Hazardous: Indicates whether a drug has been designated a hazardous compound by the National Institute for Occupational Safety & Health (NIOSH).
- 9 – Required Labs: Identifies lab tests which should be done prior to, or during, drug use.
- 10 – Contraindications: A list of reasons that a drug should NOT be used in a specific patient.
- 11 – Pregnancy: Identifies whether a drug is considered safe to use in pregnant patients.
- 12 – Compatible: Identifies IV solutions in which the drug is stable.
- 13 – Nursing Implications: Lists actions that a nurse must take to administer medication to a patient properly.
- 14 – Patient Education: Information that a nurse should relay to the patient in order for the patient to take/receive the medication in a safe and effective manner.
- 15 – Crush: Indicates whether the drug is recognized as being safe to crush or split.