Skip to content
Back of the Top 200 Drug Flashcards
All of the Important Information!
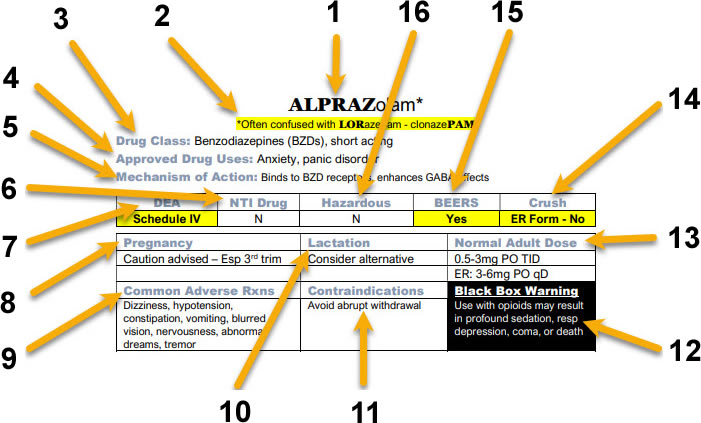
- 1 – The Generic Name of the Drug with tallMAN Lettering (where appropriate): tallMAN lettering is special labeling from The Institute for Safe Medical Practices (ISMP) for look-a-like drug names. It is used on drug labels and electronic drug orders to help prevent medical errors.
- 2 – Identification of Easily Confused Drug Names: The Institute for Safe Medical Practices (ISMP) identifies drugs that have a high error rate involving confusion of drugs with similar names.
- 3 – Drug Class: Identifies the pharmacological class to which the drug belongs.
- 4 – Approved Drug Uses: Identifies common uses for the drug.
- 5 – Mechanism of Action: Identifies the specific manner a drug exerts its effect on the body.
- 6 – NTI Drug: Identifies Narrow Therapeutic Index (NTI) drugs. NTI drugs are those in which the differences in blood levels between therapeutic and serious adverse effects is very small.
- 7 – DEA Schedule: Identifies if a drug is classified as a controlled substance by the Drug Enforcement Administration (DEA). If it is, the schedule is indicated.
- 8 – Pregnancy: Identifies whether a drug is considered safe to use in pregnant patients.
- 9 – Common Adverse Effects: A list of commonly seen adverse effects in patients
- 10 – Lactation: Identifies whether a drug is considered safe to use in patients who are breast-feeding infants.
- 11 – Contraindications: A list of reasons that a drug should NOT be used in a specific patient.
- 12 – Black Box Warning: Black box warnings are issued by the Food and Drug Administration (FDA) for drugs that been identified with serious safety risks.
- 13 – Normal Adult Dose: Lists the recommended dose for individuals without other mitigating health conditions.
- 14 – Crush: Indicates whether the drug is recognized as being safe to crush or split.
- 15 – BEERS: Indicates if the drug is potentially harmful to elderly patients by the American Geriatrics Society.
- 16 – Hazardous: Indicates whether a drug has been designated a hazardous compound by the National Institute for Occupational Safety & Health (NIOSH).